With the advancement of analytical capabilities, new chemical contaminants having no regulatory limits are being detected in the foods. Under such scenarios, risk managers of the competent authority might not have enough time to wait for the full risk assessment. Nevertheless, they must take appropriate measures to protect the public health based on the rapid risk assessment. Codex has recently formulated a guideline CXG 92-2019 to address such scenarios.
This guideline should only be applied in the conditions: (a) the contaminant is new in the food and was not previously reported; (b) it has no regulatory limit; (c) there are no specific codex guidelines or standards related to that contaminants in the foods.
Decision tree for rapid risk analysis
The codex guideline CXG 92-2019 provides the decision tree for the rapid risk analysis and is shown in the figure below:
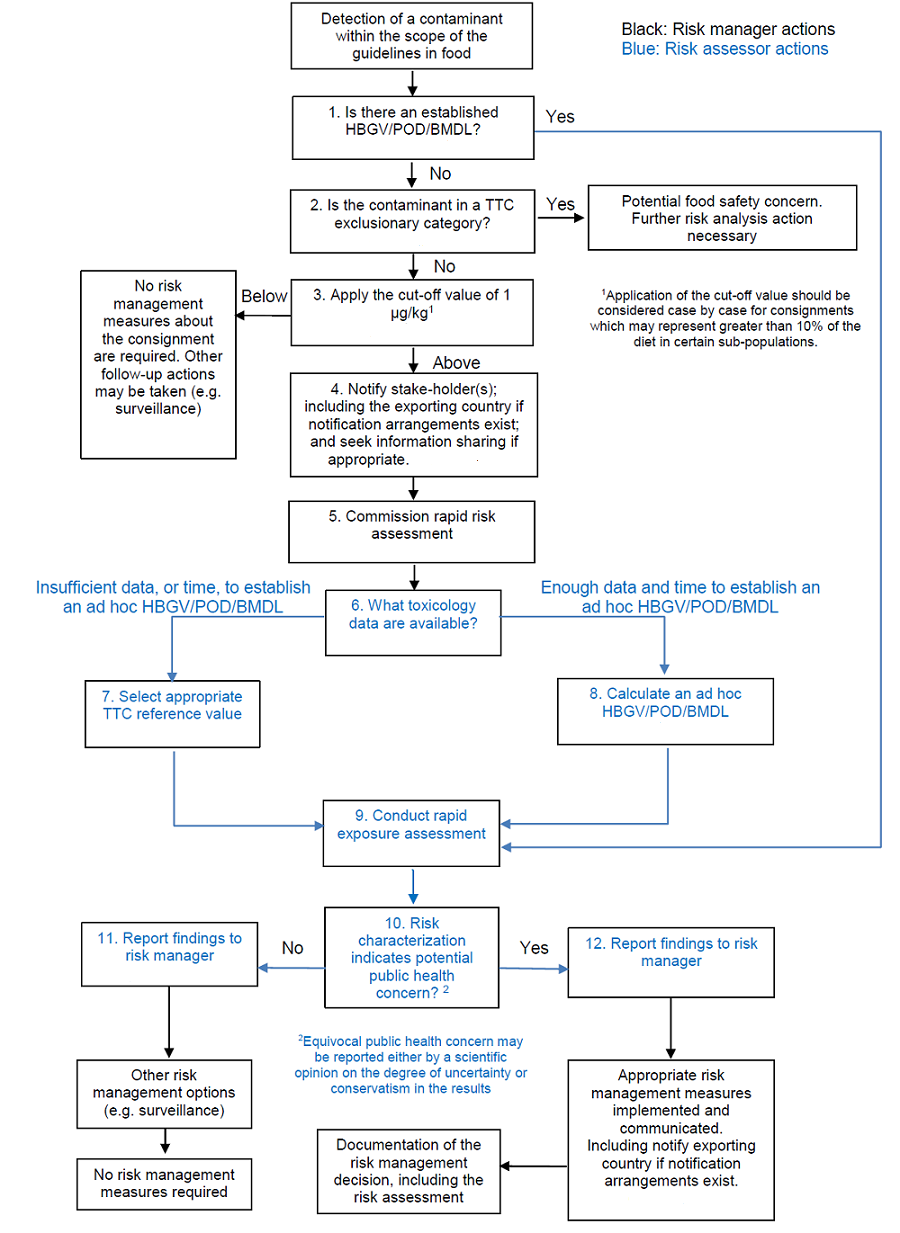
The decision tree consists of 12 steps as follows:
Step 1. Determine if the contaminants have established HBGVs, PODs or BMDLs?
Contaminants for which there are established health-based guidance values (HBGVs), toxicological points of departure (POD) or benchmark dose levels (BMDLs) can progress directly to rapid exposure assessment (Step 9) as these values enable risk characterization.
Step 2. Determine if the contaminant fall in a Threshold of Toxicological Concern (TTC) exclusionary category?
Risk managers should not follow the rapid risk analysis approach for the contaminants falling in TTC exclusionary category (such as high potency carcinogens (e.g. afloatoxins, axoxy- or N-nitroso- compounds, benzidines); unknown chemicals; inorganic chemicals; metals and organometalllics; proteins; steroids; nanomaterials; radioactive substances; organo-silicon compounds; persistent and bioaccumulative chemicals).
Step 3: Application of the cut-off value of 1 μg/kg
If the quantitative measurement of the contaminant exceeds this cut-off value, risk managers should request relevant stakeholders to share all the available information for rapid risk assessment as soon as possible.
Step 4: Request for information sharing from the competent authorities of exporting country
Risk manager should immediately request relevant food safety information (such as toxicological datasets, prior occurrence in food, food processing information, history of use etc) from the competent authorities of the exporting country. This information will be helpful during the rapid risk assessment.
Step 5: Request for rapid risk assessment
Risk managers should immediately request the risk assessor for rapid risk assessment. They should also share all the relevant food safety information collected so far.
Step 6: Toxicological data collection
The work of risk assessor starts from here. They should collect all the relevant toxicological data on the contaminant or chemically/ structurally related compounds. After the evaluation of the available toxicological data, risk assessor should make a choice of the rapid risk assessment approach. There are two possible approaches: HBGV/POD/BMDL approach or TTC approach. When enough toxicological database are available, risk assessors will chose to take HBGV/POD/BMDL approach (step 8). In the absence of such database, risk assessors will need to take TTC approach (step 7) for the rapid risk assessment.
Step 7: TTC approach (selecting appropriate TTC reference value)
If there are not sufficient toxicological data to establish a HBGV/POD/BMDL for the contaminant in food, appropriate threshold of no concern or reference value for any outcome whether genotoxic or non-genotoxic, should be selected for the contaminant based on its structural properties. The Cramer classification scheme (decision tree) is the best known approach to estimate the Threshold of Toxicological Concern (TTC). Some software (e.g. Toxtree) can be very helpful for using the TTC approach.
Step 8: Establishment of a HBGV/POD/BMDL toxicological value
When sufficient toxicological data are available for the contaminant in food, risk assessor should try to establish an ad-hoc HBGV/POD/BMDL value. The risk characterization should be carried out using such toxicological value.
Step 9: Conduct rapid exposure assessment
A rapid estimation of the exposure of the contaminant from the consumption of the food should be carried out based on the estimated consumption and contamination data. The exposure assessment could be done using deterministic approach. The probabilistic exposure assessment might not be feasible during rapid risk analysis.
Step 10: Rapid risk characterization
By comparing the estimated exposure with the toxicological data (developed using either TTC approach (step 7) or using HBGV/POD/BMDL toxicological value (step 8)), a rapid risk characterization should be performed. Appropriate measures such as comparison with ADI (accetable daily intake), TDI (tolerable daily intake), calculation of Margin of Exposure (MOE) etc can be used for risk characterization. The risk characterization should determine the level of risk from the exposure of the contaminant from the food source. The risk assessors should clearly mention the assumptions made during these calculations. If the level of risk is high, risk assessors can also provide the appropriate mitigation strategies to reduce the risk.
Step 11 & 12: Reporting to risk managers
The risk assessor should provide the results of the risk analysis including the information on assumption and uncertainties to the risk managers. The reporting should be done in a timely manner so that appropriate risk management approach can be taken. The reporting should be done in a clear and consistent manner so that risk managers can take appropriate decisions.
Step 13: Decisions by risk manager
The risk manager should determine the risk management response based on the results of the rapid risk assessment provided by the risk assessor. The risk management decision should be science and evidence based. In addition, targeted surveillance program should be started to gain more information on the contamination and consumption data. This will help to carryout detailed risk assessment in future and will also help to determine the recurrence of instances of detection of the contaminant in food.
The risk management approach taken based on the rapid risk analysis should be reviewed timely based on the updated information. When necessary, risk managers can request the risk assessors for the detailed risk assessment. Appropriate regulatory limit can be set at the national and international level for that contaminant.
RISK COMMUNICATION
The codex guideline entitled “The principles and guidelines for the exchange of information between importing and exporting countries to support the trade in food (CXG 89-2016)” could be applied during the exchange of food safety information between the competent authorities. In addition, other relevant stakeholders (such as other competent authorities, relevant government organizations, International food safety related organizations, consumers etc) have high level of interest in the information on the presence of contaminants in food. The outcomes of the risk assessment and risk management could be useful for other competent authorities as well. Therefore, appropriate risk communication related to both the risk assessment and risk management should be carried out.
References
CXG 89- 2016. The Principles and Guidelines for the Exchange of Information between Importing and Exporting Countries to Support the Trade in Food.
CXG 92-2019. Guidelines for rapid risk analysis following instances of detection of contaminants in food where there is no regulatory level.





No comments:
Post a Comment